Answer:
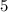 mg parent isotope would be left after 2 million years
mg parent isotope would be left after 2 million years
Step-by-step explanation:
It is given that half life of parent isotope is equal to one million years.
Half life period indicates the number of years required by an isotope to reduce its quantity by half.
Amount of parent isotope left after first half life period i,e after
 million years
million years
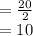 mg
mg
Amount of parent isotope left after second half life period i,e after
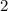 million years
million years
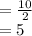 mg
mg