Answer: It is a type of neutralization reaction.
Explanation:
Synthesis reaction: In this reaction, two or more simple substances combine directly to form a complex substance.
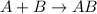
Decomposition reaction: In this reaction, a single compound breaks down into two or more compounds.
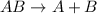
Single displacement reaction: In this reaction, one element reacts with a compound to produce another element and another compound.
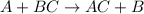
Neutralization reaction: In this reaction, acid reacts with base to produce a salt and water molecule.
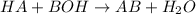
For a given reaction,
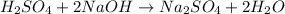
 is a acid
is a acid
NaOH is a base
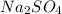 is a salt
is a salt
As the given reaction is a type of Neutralization Reaction.