to find moles from any number of atoms, always divide by the constant called Avogadro's Number. This number is representative of the number of atoms or molecules in a mole of any substance and is equal to about
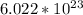
.
So, for this problem, we take the number of atoms of oxygen and divide by Avogadro's Number.
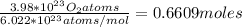 Answer: 0.661 moles
Answer: 0.661 moles (after rounding)
Make sense?