Answer: 10 grams of Hydrogen gas will be produced theoretically.
Explanation: The equation for the reaction between zinc metal and hydrochloric acid is:
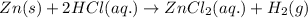
As HCl is present in excess, therefore limiting reagent is Zinc metal as it limits the formation of product.
By stoichiometry,
1 mole of Zinc metal produces 1 mole of Hydrogen gas, therefore
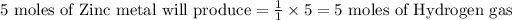
Amount of Hydrogen gas produced can be calculated by:
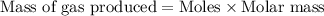
Molar mass of Hydrogen = 2 g/mol
Mass of hydrogen gas produced = (5 moles )× (2 g/mol)
Mass of hydrogen gas produced = 10 grams