Answer: Rate = 0.14 kg/s
Step-by-step explanation: The Haber reaction is demonstrated below in the balanced equation
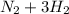 →
→
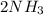
To determine the rate of ammonia production, we need to determine the amount of nitrogen gas is being consumed.
As it is a gas, we can find moles by using Ideal Gas Law, whose formula is
PV = nRT
P is pressure
V is volume
n is moles
R is universal gas constant
T is temperature in Kelvin (K)
For the nitrogen gas, temperature will be
T = 273 + 17.2
T = 290.2 K
Solving for moles:
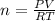
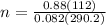
n = 4.14 moles
In the Haber reaction, it is consumed 4.14 moles per second of nitrogen gas.
From the balanced equation, we know 1 mol of nitrogen gas produces 2 moles of ammonia, so
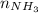 = 2(4.14)
= 2(4.14)
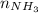 = 8.48 moles
= 8.48 moles
Number of moles (n) is mass in grams divided by molar mass of the compound:
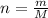
Molar mass of ammonia is M = 17.031 u, so mass produced is
m = n.M
m = 8.48(17.031)
m = 144.42 g
In kilograms, m = 0.14 kg
When consuming N₂ at a rate of 4.14 moles/s, it will be produced ammonia at a rate of 0.14 kg/s