Answer: The correct answer is option (A).
Step-by-step explanation:
Given mystery ions:
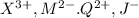
Positive charged ions will form compounds with negatively charged ions.So compounds possible from these ions are:
1)
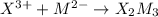
2)
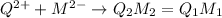
(Integer in subscript of the ion will get cancelled by dividing with 2.)
3)
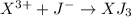
4)
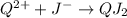
All the desired compounds enlisted above are possible to be formed. the from the option given the formula would not be correct for an ionic compound will be
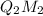 that is option (A).
that is option (A).
Hence, the correct answer is option (A).