Answer:
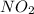
Explanation: The empirical formula of a compound is the simplest whole number ratio of atoms in the compound. In
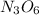 , the ratio of N atoms to O atoms is 3:6, which can be reduced to 1:2. Using the reduced ratio, put new subscripts on the compound:
, the ratio of N atoms to O atoms is 3:6, which can be reduced to 1:2. Using the reduced ratio, put new subscripts on the compound:
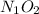 . Since subscript of 1 on the N is implied, no subscript needs to be written on the N, and the empirical formula is simply
. Since subscript of 1 on the N is implied, no subscript needs to be written on the N, and the empirical formula is simply
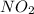 .
.