Answer:
The best option is for the following option m = 15 [g] and V = 5 [cm³]
Step-by-step explanation:
We have that the density of a body is defined as the ratio of mass to volume.
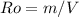
where:
Ro = density = 3 [g/cm³]
Now we must determine the densities with each of the given values.
For m = 7 [g] and V = 2.3 [cm³]
![Ro=7/2.3\\Ro=3.04 [g/cm^(3) ]](https://img.qammunity.org/2022/formulas/physics/high-school/3d3g4twc0h9r6cfkx5d24cbr29swwr0907.png)
For m = 10 [g] and V = 7 [cm³]
![Ro=10/7\\Ro=1.42[g/cm^(3) ]\\](https://img.qammunity.org/2022/formulas/physics/high-school/ioa6y61ownp908z8oaqt24337nwcx59w6r.png)
For m = 15 [g] and V = 5 [cm³]
![Ro=15/5\\Ro=3[g/cm^(3) ]\\](https://img.qammunity.org/2022/formulas/physics/high-school/i1wv7vg27fh3yemycu693c3e0y3qrudlbb.png)
For m = 21 [g] and V = 8 [cm³]
![Ro=21/8\\Ro=2.625[g/cm^(3) ]\\](https://img.qammunity.org/2022/formulas/physics/high-school/ziur4lk4w0jmi9nr0ds3ctz1f62zz6acmw.png)