You are decreasing "disorder" by building one molecule out of two. The entropy will be negative. So, we know "a" and "c" are incorrect.
ΔS°reaction = (∑"n" moles of products × the ΔS° of the products) - (∑ "n" moles of reactants × the ΔS° of the reactants)
(I looked these up. I hope they are the same as your table.)
PRODUCT ENTHALPIES
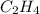
ΔS° 219.4 (J/K mol)
REACTANT ENTHALPIES
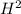
ΔS° 130.58 (J/K mol)
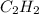
ΔS° 200.8(J/K mol)
Now, we solve:
ΔS°reaction = (∑"n" moles of products × the ΔS° of the products) - (∑ "n" moles of reactants × the ΔS° of the reactants)
ΔS°= ((n* 219.4))-((n*200.8)+(n*130.58))
(because all the molec. have a coefficient of 1 in front of them, "n"= 1 for all of them.)
ΔS°= (219.4)-((200.8)+(130.58))
ΔS°= (219.4)-(331.38)
ΔS°= (-111.98)
rounded to -112
Answer: d. - 112 J/K mol
Comment with any further questions! Hope it all made sense!