Answer:
12.09g of water are produced.
Step-by-step explanation:
1st) From the chemical equation we know that 2 moles of C4H10 (butane) react with oxygen to preoduce 10 moles of H20 (water) and CO2.
It is necessary to use the molar mass of C4H10 (58.12g/mol) and H2O (18g/mol) to convert moles to grams:
• C4H10 conversion:
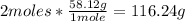
• H2O conversion:
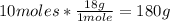
Now we know that 180g of water are produced from 116.24g of butane.
2nd) With a mathematical rule of three and the stoichiometry of the reaction we can calculate the mass of water produced when 7.81g of butane reacts with excess oxygen:
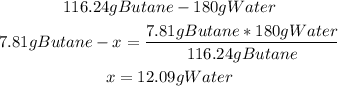
Finally, 12.09g of water are produced.