Answer:
Exothermic reaction for the HCl, endothermic reaction for the water
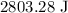
Step-by-step explanation:
Heat was lost by HCl as its temperature lowered, so it was an exothermic reaction for the HCL.
Heat was gained by water as its temperature increased, so it was an endothermic reaction for the water.
m = Mass of water = 100 g
c = Specific heat of water =
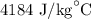
 = Change in temperature of water =
= Change in temperature of water =
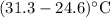
Heat is given by
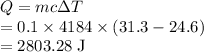
Heat gained by water is
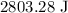 .
.