Answer:
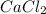
Step-by-step explanation:
Hello,
Calcium is alkaline-earth metal located at the group IIA, which means that gains two electrons when bonded with another element, thus, its oxidation state is +2. However, the halogen (group VIIA element) chlorine in the form of chloride ions loses one electron, hence is oxidation state is -1. In such a way, when they gather in one compound, the resulting formula turn out into:
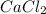
Which accounts for calcium chloride.
Best regards.