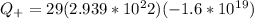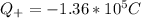Answer:
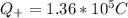
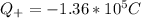
Step-by-step explanation:
From the question we are told that
Mass of old penny
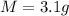
Generally the number of moles in a penny is given mathematically as
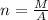
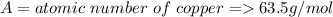
Therefore
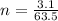
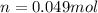
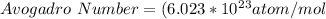
Therefore
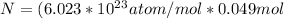

Generally the Total positive charge of the copper is given by
Since its the 29th atom of the periodic table
29 protons
29 electrons
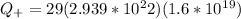
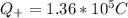
Generally the Total negative charge of the copper is given by