Given,
Mass of the sample, m=47 g= 0.047 kg
The initial temperature, T₁=80 °F=299.8 K
The final temperature of the sample, T₂=149 °F=338.2 K
Therefore the total raise in the temperature,
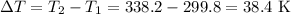
The specific heat capacity of the aluminum, c=0.903 J/(g · °C)= 903 J/(kg · K)
The heat required to raise the temperature of a sample by a temperature of ΔT is given by,
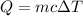
On substituting the known values in the above equation,
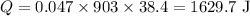
Therefore the heat required to raise the temperature of the sample from 80 °F to 149 °F is 1629.7 J