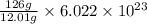Answer: The equivalence factor set that should be used is
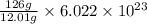
Step-by-step explanation:
Molar mass of a substance is defined as the mass of one mole of a substance.
We know that:
Molar mass of carbon atom = 12.01 g/mol
Given mass of carbon atom = 126 grams
Applying unitary method:
12.01 grams of carbon occupies 1 mole
So, 126 grams of carbon will occupy =
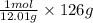
According to mole concept:
1 mole of an element contains
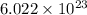 number of atoms
number of atoms
So,
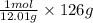 of carbon will contain =
of carbon will contain =
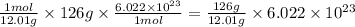 number of atoms
number of atoms
Hence, the equivalence factor set that should be used is