Answer:
Option (B) is correct.
The mass of the reactants equals the mass of the products.
Explanation:
According to law of conservation of mass :
In a balanced chemical equation, the mass of reactants is always equal to the mass of products.
Here, Water
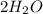 is the reactant which has 4 units of Hydrogen and 2 units of oxygen.
is the reactant which has 4 units of Hydrogen and 2 units of oxygen.
and
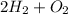 are products which also has 4 units 4 units of Hydrogen and 2 units of oxygen.
are products which also has 4 units 4 units of Hydrogen and 2 units of oxygen.
Thus, The mass of the reactants equals the mass of the products.
Thus, option (B) is correct.