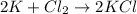Answer: KCl
Explanation: Potassium [K] has an atomic no of 19 and the electronic configuration is:
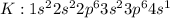 tends to get stable by losing one electron and forming
tends to get stable by losing one electron and forming
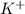
Chlorine [Cl] has atomic no of 17 and thus the electronic configuration is :
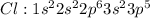 tends to get stable by gaining one electron and forming
tends to get stable by gaining one electron and forming
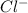
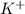 and
and
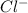 attract each other and form an ionic bond. As we have a chlorine molecule that is two atoms of chlorine are present, 2 atoms of potassium will react to form 2 moles of KCl.
attract each other and form an ionic bond. As we have a chlorine molecule that is two atoms of chlorine are present, 2 atoms of potassium will react to form 2 moles of KCl.