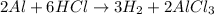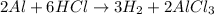
Step-by-step explanation:
In order for the law of conservation of mass to be valid for this reaction, we must balance the atoms of each element so that it is the same in the reactant side and the product side.
In this case, we immediately see that the hydrogen (H) is not balanced: we have 3 on the left and 2 on the right. So, we must put a factor 2 on the
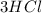 on the left and a factor 3 on the
on the left and a factor 3 on the
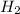 on the right. Now the Cl is no longer balanced (6 on the left and 3 on the right), so we must put a factor 2 in front of
on the right. Now the Cl is no longer balanced (6 on the left and 3 on the right), so we must put a factor 2 in front of
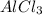 . And to balance the Al, we must add a coefficient 2 in front of the Al on the right.
. And to balance the Al, we must add a coefficient 2 in front of the Al on the right.
Summarizing everything, the balanced reaction is: