Answer:
%
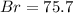 %
%
Step-by-step explanation:
Hello,
From the AgBr precipitate, one could compute one can compute the bromine grams as follows:
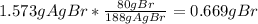
As long as there was an excess of silver nitrate, one knows that into the 1.573 g of AgBr, 0.669 g correspond to the bromine that was initially contained into the 0.8838-g sample, thus, the percent is computed as follows:
%
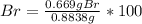 %
%
%
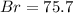 %
%
Best regards.