Answer : The reaction shows the formation of hydronium ion is,
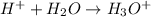
Explanation :
The given reactions are:
(1)
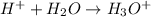
In this reaction, hydrogen ion react with water to give hydronium ion as a product.
(2)
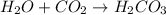
In this reaction, water react with carbon dioxide to give carbonic acid as a product.
(3)
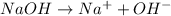
In this reaction, sodium dissociate to give sodium ion and hydroxide ion as a products.
(4)
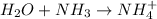
In this reaction, water react with ammonia to give ammonium ion as a product.
From this we conclude that, only reaction 1 shows the formation of hydronium ion. While the reactions do not shows the formation of hydronium ion.