Answer:
The final temperature of water = 52.6 C
Step-by-step explanation:
The heat (q) lost or gained by a substance of mass m corresponding to a temperature change from T1 to T2 degrees is given as:
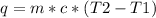 -----(1)
-----(1)
where c = specific heat of the substance
For water, c = 4.18 J/gC
In the given situation:
Heat lost by 70.3 g of water = Heat gained by 100.0 g of water
Based on equation (1) and considering that heat lost is negative:
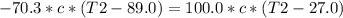
Solving the above equation gives:
T2 = 52.6 C