Answer: Option (c) is the correct answer.
Step-by-step explanation:
Neon has atomic number 10 and its electronic distribution is 2, 8. As it has completely filled valence shell therefore, it does not need to gain or lose an electron.
Hence, neon is stable in nature and does not form bonds with any other atom.
Whereas atomic number of gold is 79 and its electronic configuration is
![[Xe] 4f^(14)5d^(10)6s^(1)](https://img.qammunity.org/2018/formulas/chemistry/high-school/5rvng7ngv0vp19zpurgavv661gzk070cv2.png) . Hence, in order to attain stability, it loses one electron and thus, it is likely to form a bond.
. Hence, in order to attain stability, it loses one electron and thus, it is likely to form a bond.
Oxygen atom has atomic number 16 and its electronic distribution is 2, 8, 6. Hence, to attain stability it needs 2 electrons. Hence, it is likely to form a bond.
Magnesium has 2 valence electrons and chlorine has 7 valence electrons. So, they combine chemically to form
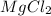 .
.
Thus, we can conclude that out of the given options neon atoms are not likely to form bonds.