angular momentum quantum number:
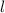
magnetic quantum number:
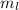
The magnetic quantum number
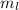
may take values
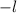
through
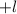
.
In this case
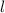
is 2, so the magnetic quanum number may take values - 2, - 1, 0, +1 and + 2.
That is 5 possible values.Therefore, the answer is 5.