Step-by-step explanation:
An element that gains one electron will tend to acquire a negative charge whereas an element that tend to lose an electron will acquire a positive charge.
Similarly, an element losing two electrons will acquire a 2+ charge.
For example, calcium is a group II element and has 2 valence electrons. Therefore, to attain stability it loses two electrons and thus changes into
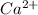 ion.
ion.
Hence, we can conclude that an element of Group II loses two electrons in the process of a chemical combination then its ionic charge will be +2.