Answer: In this reaction, a precipitate is being formed.
Step-by-step explanation:
A chemical change can be determined by the following indicators:
- A characteristic odor
- Color change
- Formation of bubbles
- Formation of precipitate
- Evolution of gas
When magnesium ions and hydroxide ions react in aqueous state, it leads to the formation of a white colored solid of magnesium hydroxide.
In the above reaction, a precipitate is being formed and hence the reaction is considered as a precipitation reaction.
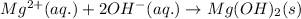
Therefore, in this reaction, the chemical change is that the precipitate is getting formed.