Answer:
final relaxed state is m = 3
Step-by-step explanation:
As we know that due to transition of electron the wavelength emitted is given as
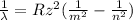
now we know that
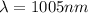
now we have
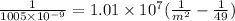
now by solving above equation for m we will have
m = 3
so the final relaxed state is m = 3