Answer: Option (d) is the correct answer.
Step-by-step explanation:
An equation will be said balanced equation when there are equal number of atoms on both reactant and product side.
For example,
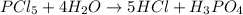
Number of atoms on reactant sides are as follows.
P = 1
Cl = 5
H = 8
O = 4
Number of atoms on product side are as follows.
P = 1
Cl = 5
H = 8
O = 4
Thus, there are equal number of atoms on both reactant and product side. Hence, this equation is balanced.