Step-by-step explanation:
Formula used to calculate average atomic mass follows:
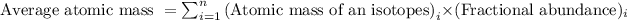
Let the fractional abundance of B-10 isotope be 'x'. So, fractional abundance of B-11 isotope will be '1 - x'
For B-10 isotope:
Mass of B-10 isotope = 10.0129 amu
For Li-7 isotope:
Mass of B-11 isotope = 11.0931 amu
1) Average atomic mass of boron = 10.807 amu
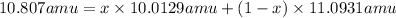
x= 0.2648
The percent abundances B-10 isotope = 0.2648 × 100 =26.48%
The percent abundances B-11 isotope = 100% - 26.48% =73.51%
2) Average atomic mass of boron = 10.819 amu
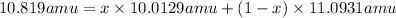
x= 0.2537
The percent abundances B-10 isotope = 0.2537 × 100 =25.37%
The percent abundances B-11 isotope = 100% - 25.37% =74.63%