The question requires us to calculate the density of a pebble, given the mass (in grams) and volume (in cubic centimeters) of the object.
The information provided by the question is as follows:
mass = m = 64.2 g
volume = v = 20.7 cm3
To calculate the density of an object (d), we need to divide its mass (m) by its volume (v). If we do this operation using mass in g and volume in cm3, we will have the density in g/cm3 units.
On the other side, if we calculate the density using mass in units of ounces and the volume in units of cubic inches, the resultant unit will be ounces/cubic inches (which is the required unit by the question).
Thus, before calculating the density, we need to convert the values given.
To do that, we need to keep in mind that 1 gram corresponds to 0.035274 ounces and 1 cubic centimeter equals to 0.0610237 cubic inches:
1 gram ---------- 0.035274 ounces
1 cubic centimeter ---------- 0.0610237 cubic inches
Using the relations above, we calculate how many ounces are there in 64.2g:
1 g ---------- 0.035274 ounces
64.2 g ---- x
Solving for x (multiplying 64.2 by 0.035274), we have 2.26 ounces (2.26 oz)
For the volume of 20.7 cm3, we use the following scheme:
1 cm3 ---------- 0.061023 cubic inches
20.7 cm3 ---- y
Solving for y (multiplying 20.7 by 0.061023), we have 1.26 cubic inches (1.26 in3)
Next, we calculate the density by dividing the mass found (2.26 oz) by the volume (1.26 in3):
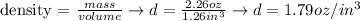
Therefore, the density of the given pebble is 1.79 oz/in3 (or 3.10 g/cm3).