Given:
ρ = 2.95 g/L = 2.95 kg/m³, the density
T = 32°C = 32+273 K = 305 K, the temperature
p = 860 mm Hg = (860/760) atm = 1.1316 atm
= 1.1316*101325 Pa
= 1.14657 x 10⁵ Pa
The ideal gas law is
p = ρ*(R/M)*T
where
R = 8.314 J/(mol-K), the gas constant
M = molar mass, kg/mol
The molar mass is
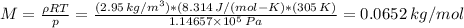
The molar mass is 0.0652 kg/mol = 65.2 g/mol
Answer: 65.2 g/mol