Answer: The given statement is false.
Step-by-step explanation:
Electronic configuration of calcium is
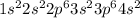 , that is, there are two extra electrons. So, in order to attain stability calcium loses two electrons readily.
, that is, there are two extra electrons. So, in order to attain stability calcium loses two electrons readily.
Whereas electronic configuration of copper is
![[Ar]4s^(1)3d^(10)](https://img.qammunity.org/2018/formulas/chemistry/middle-school/s79wpdrj1rao88t7llzuks4exqzcureo1y.png) . Since, it has completely filled d-orbital so, it is stable in nature and thus, does not react readily.
. Since, it has completely filled d-orbital so, it is stable in nature and thus, does not react readily.
Therefore, we can conclude that reactivity of calcium is more than copper. That is why, the given statement is false.