Answer:
(a)
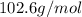
(b) Rubidium
Step-by-step explanation:
Hello,
This titration is carried out by assuming that the volume of base doesn't have a significant change when the mass is added, thus, we state the following data a apply the down below formula to compute the molarity of the base solution:
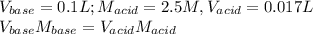
Solving for the molarity of base we've got:
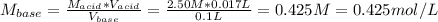
Now, we can compute the moles of the base as:
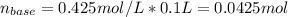
(a) Now, one divides the provided mass over the previously computed moles to get the molecular mass of the unknown base:
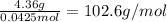
(b) Subtracting the atomic mass of oxygen and hydrogen, the metal's atomic mass turns out into:
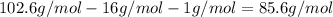
So, that atomic mass dovetails to the Rubidium's atomic mass.
Best regards.