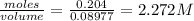Answer:
Molarity is 2.272M
Step-by-step explanation:
The mass % of phosphoric acid is 20.0%
It means in 100 g of solution there is 20g of phosphoric acid in it.
Molarity = moles of solute dissolved per litre of solution.
So we need volume of solution
Volume can be calculated from mass and density as
Volume = mass / Density = 100/1.114 = 89.77 mL = 0.08977 L
Moles of solute = mass of solute / molar mass of solute
moles of solute = 20g / 98 = 0.204
molarity =