Answer: Option (d) is the correct answer.
Step-by-step explanation:
Noble gases are the gases of group 18 which are helium, neon, argon, krypton, xenon and radon.
For example, electronic configuration of neon is
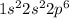
These elements have completely filled shells so, they do not need to gain or lose an electron as they are stabilized.
As a result, noble gases become unreactive in nature.
Thus. we can conclude that noble gases is the name of the group of elements that are unreactive gases.