Step-by-step explanation:
Coefficients are the number present in front of an atom or molecule in a chemical reaction.
For example,
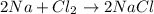
In this reaction 2 in front of Na in reactant side shows 2 moles of sodium atoms are participating. Whereas 2 in front of NaCl in product side shows that 2 moles of sodium chloride is formed.
Therefore, we can conclude that coefficients in a balanced chemical equation tell you the moles of each substance involved in the reaction.