Answer:
The change in internal energy will be 25 J.
Step-by-step explanation:
Given that,
Work done = 25 J
Using first law of thermodynamics
The internal energy is sum of the heat and work done.
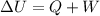
Where,
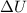 = Change in internal energy
= Change in internal energy
Q = heat
W = work done
In adiabatic process, the heat does not change, it remains constant.
So the heat Q = 0
Therefore, The change in internal energy will be
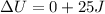
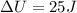
Hence, The change in internal energy will be 25 J.