The combined gas law is
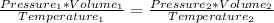
with 1 and 2 both being anything with pressure, volume, and temperature. Since pressure is on the top and temperature is on the bottom, they are inversely related, meaning that when the temperature gets high the pressure goes low. In addition, since pressure and volume are both on the top, when volume goes down pressure goes down too. Therefore, since A and C are right, D is the correct answer