Answer: -
The limiting reactant is Al.
Explanation: -
Number of moles of O₂ = 6.7 moles
The balanced chemical equation for this process is
4 Al + 3 O₂ → 2Al₂O₃
From the balanced reaction we see
3 mol of O₂ would react with 4 mol of Al
6.7 mol of O₂ would react with
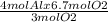
= 8.933 mol of Al
But the number of moles of Al present is only 4.5 mol.
Hence the limiting reagent is Al.