Given that the half life of 3H is 12.3 years.
The amount of substance left of a radioactive substance with half life of
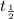
after t years is given by
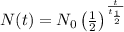
Therefore, the number of years it will take for 18% of the original tritinum to remain is given by
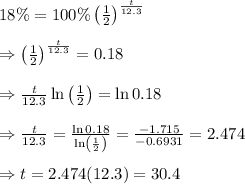
Therefore, the number of
years that the time could be read at night is 30.4 years.