Given:
m = 0.240 kg = 240 g, the mass of O₂
V = 3.10 L = 3.10 x 10⁻³ m³, the volume
Because the molar mass of oxygen is 16, the number of moles of O₂ is
n = (240 g)/(2*16 g/mol) = 7.5 mol
As an ideal gas,
p*V = nRT
or
V = (nRT)/p
where R = 8.314 J/(mol-K)
When
p = 0.910 atm = (0.910 atm) * (101325Pa/atm) = 92205.75 Pa
T = 27 °C = (27 + 273) K = 300 K
then the volume is
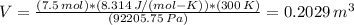
V = (0.2029 m³)*(10³ L/m³) = 202.9 L
Answer: 203 liters