Answer:
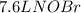
Step-by-step explanation:
Hello,
In this case, since no temperature and pressure are known, one could develop the stoichiometric relationship for 1 mole of
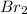 per 2 moles of
per 2 moles of
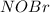 in terms of volume as shown below because of the Avogadro's law (change in mole proportional to the change in volume at constant both pressure and temperature):
in terms of volume as shown below because of the Avogadro's law (change in mole proportional to the change in volume at constant both pressure and temperature):
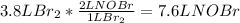
Best regards.