Ethanoic (Acetic) acid is a weak acid and do not dissociate fully. Therefore its equilibrium state has to be considered here.
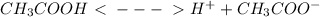
In this case pH value of the solution is necessary to calculate the concentration but it's not given here so pH = 2.88 (looked it up)
pH = 2.88 ==>
![[H^(+)]](https://img.qammunity.org/2018/formulas/chemistry/high-school/tixcvmiul05vtrn8s7qstptt69zx1u6brh.png)
=
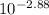
= 0.001

The change in Concentration Δ
![[CH_(3)COOH]](https://img.qammunity.org/2018/formulas/chemistry/college/o2pzvta5n41rycid34zwdt1othw3hzvtgg.png)
= 0.001

CH3COOH H+ CH3COOH
Initial


0 0
Change

-0.001 +0.001 +0.001
Equilibrium


- 0.001 0.001 0.001
Since the
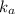
value is so small, the assumption
![[CH_(3)COOH]_(initial) = [CH_(3)COOH]_(equilibrium)](https://img.qammunity.org/2018/formulas/chemistry/college/s353byj4yvlgylgq1jqyd05w8wnb8aot3n.png)
can be made.
![k_(a) = [tex]= 1.8*10^(-5) = ([H^(+)][CH_(3)COO^(-)])/([CH_(3)COOH]) = (0.001^(2))/(x)](https://img.qammunity.org/2018/formulas/chemistry/college/hps6zn8h54men7ozvt52zvygcd0632gf8q.png)
Solve for x to get the required concentration.
note: 1.)Since you need the answer in 2SF don&t round up values in the middle of the calculation like I've done here.
2.) The ICE (Initial, Change, Equilibrium) table may come in handy if you are new to problems of this kind
Hope this helps!