Answer : The sample of
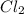 effuses in, 350 seconds.
effuses in, 350 seconds.
Solution : Given,
Effusion time of
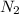 = 220 s
= 220 s
Molar mass of
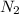 = 28 g/mole
= 28 g/mole
Molar mass of
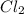 = 71 g/mole
= 71 g/mole
Rate of effusion : It is defined as the volume of gas effused in a given time 't'.
Formula used :
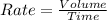
Or,
Rate of effusion : It is defined as the rate of effusion is directly proportional to the square root of the mass of the gas.
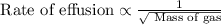
From the two expressions, we conclude that the relation between the time and the mass of gas is,
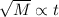
or,
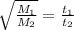 .........(1)
.........(1)
where,
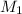 = molar mass of
= molar mass of
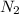 gas
gas
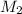 = molar mass of
= molar mass of
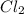 gas
gas
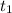 = time of effusion of
= time of effusion of
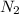 gas
gas
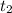 = time of effusion of
= time of effusion of
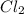 gas
gas
Now put all the given values in equation (1), we get
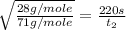
By rearranging the terms, we get
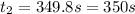
Therefore, the sample of
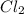 effuses in, 350 seconds.
effuses in, 350 seconds.