Answer:
Angle between the carbon-sulfur bond and the carbon-nitrogen bond in the thiocyanate ion
 is 180°.
is 180°.
Step-by-step explanation:
The hybridization of carbon atom is sp hybridized. The shape of the sp hybridized carbon is linear which means that angle between sulfur carbon a bond and carbon nitrogen bond is 180 °.
Where as
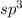 hybridized carbon has tetrahedral shape with an angle of 109.5°.
hybridized carbon has tetrahedral shape with an angle of 109.5°.
Where as
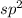 hybridized carbon has trigonal planar with an angle of 120°.
hybridized carbon has trigonal planar with an angle of 120°.