Step-by-step explanation:
A pure substance is defined as the substance that contains same type of atoms or atoms of one type of molecule.
A pure substance will always have a fixed melting and boiling point.
For example, at room temperature bromine is a liquid and its boiling point of
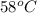 and a melting point of
and a melting point of
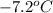 .
.
Hence, bromine is a pure substance.
Whereas when two or more different substances are mixed together irrespective of their ratio by mass then they are known as mixtures. And, mixtures always show a range of temperature for their melting and boiling point.
Hence, bromine is not a mixture.