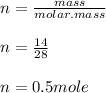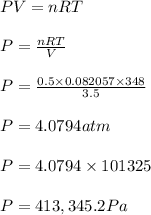Answer:
P = 4.0794 atm or 413,345.2 Pa
Step-by-step explanation:
Given
Mass m = 14.0 g
Volume 3.5 L
Temperature T = 75° C = 75 + 273 = 348 K
Known Values
Molar mass of CO = 28 g/mol
Universal gas constant R =
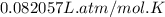
Solution
Number of moles in 14 g of CO is