Given :
Mass of Ba(OH)₂ , m = 35.5 g.
Volume of mixture, V = 325 mL = 0.325 L.
To Find :
The molarity of a solution.
Solution :
We know, molarity of a solution is given by :
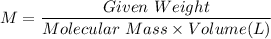
We know, molecular mass of Ba(OH)₂ is given by :
M.M. = 171 g/mol
Putting all these values in given equation, we get :
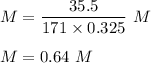
Hence, this is the required solution.