Answer:
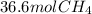
Step-by-step explanation:
Hello,
The carried out chemical reaction is:
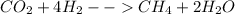
With the given moles of carbon dioxide, one computes the required moles of methane by applying the following stoichiometric relationship based on the undergoing chemical reaction.
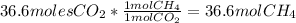
Best regards