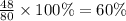Answer: 80 g
Step-by-step explanation:Molar mass of the compound is the mass of 1 mole of compound which is the sum of masses of each element.
Mass of 1 mole of compound=mass of nitrogen + mass of hydrogen+ mass of oxygen= 28+4+48 = 80 g.
Percentage by mass of nitrogen=
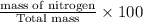
Percentage by mass of nitrogen=
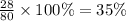
Percentage by mass of hydrogen=
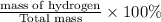
Percentage by mass of hydrogen=
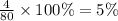
Percentage by mass of oxygen=
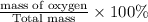
Percentage by mass of oxygen=