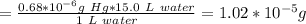Answer:
Amount of mercury is 1.0*10⁻⁵ g
Step-by-step explanation:
Given:
Mercury content of stream = 0.68 ppb
volume of water = 15.0 L
Density of water = 0.998 g/L
To determine:
Amount of mercury in 15.0 L of water
Calculation:
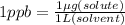
where 1 μg (micro gram) = 10⁻⁶ g
0.68 ppm implies that there is 0.68 *10⁻⁶ g mercury per Liter of water
Therefore, the amount of mercury in 15.0 L water would be: